Von Willebrand Factor (VWF): Biochemical and molecular characteristics
The VWF is an adhesive glycoprotein present in different cell types such as platelets, endothelial cells, megacariocites, and so on. It is synthesized in a precursor molecule form that, once splited, releases an amino acid propeptide of 741 aa leading to a large mature subunit (2,050 amino acids). Through a disulfide bond, this subunit is capable of forming multimeric structures with variable sizes reaching the 100 monomers and a molecular weight of the order of 10,000 kilodaltons (Figure 1).
The transcript of the VWF gene generates about 9 kilobases of mRNA that encodes a precursor of 2,813 amino acids known as pre-pro-VWF. This mRNA is transcribed from a single gene located on the telomeric end of the short arm of chromosome 12 (position 12p12). The VWF gene extends about 178 kb in the genome and contains a total of 52 exons, with measures ranging between 40 and 1,400 base pairs (bp), making it one of the largest and most complex genes described in human (Figure 1) [1, 2].
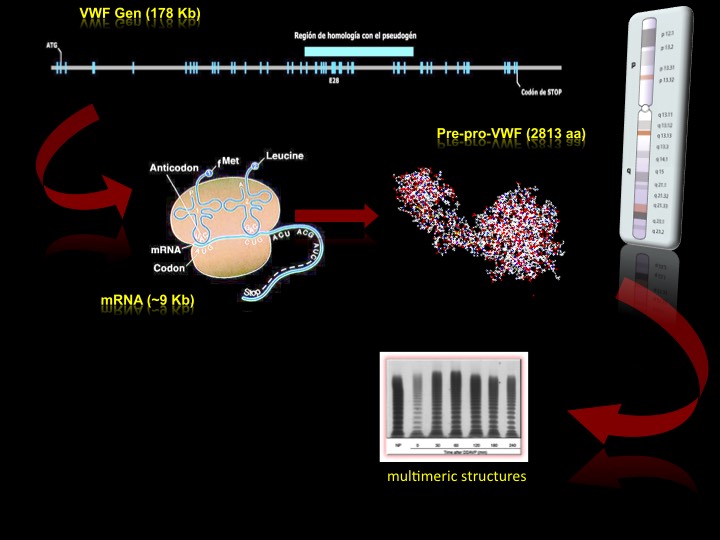
The VWF gene is located on chromosome 12, it is 178 kb long and consists on 52 exons that encode the mRNA. Once this mRNA is translated, it is obtained an initial protein consisting of up to 2813 aa, through a series of post-traduccional processes is modified to produce a VWF mature subunit of 2,050 aa. These subunits are secreted abroad and are organized in multimeric structures through the formation of disulfide bridges. These multimers have the adhesive function
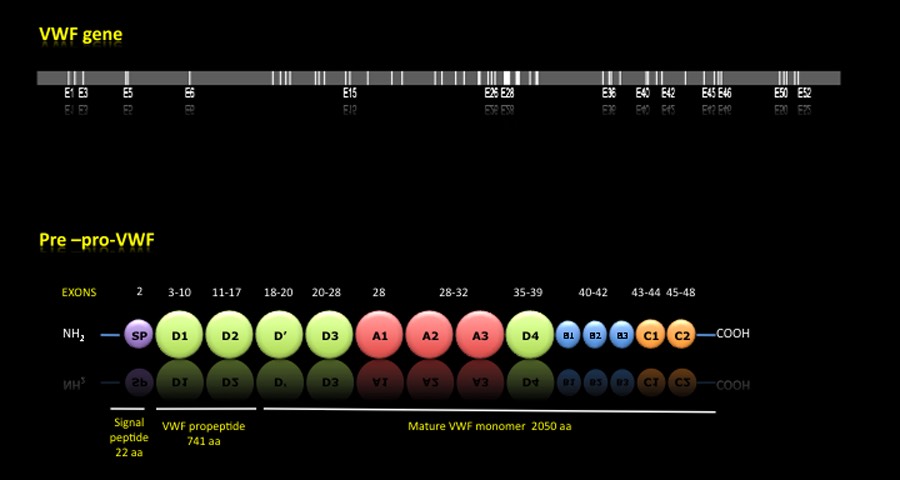
PRE-PRO-VWF:
Once the mRNA is translated, it is formed a premature protein called Pre-pro-VWF made up of a signal peptide of 22 aa, a propeptide of 741 aa and a mature subunit of 2050 aa. The pro-VWF, formed by the propeptide and mature subunit, is composed of 4 types of domains that are repeated along the protein and carrying out the various functions that allow the platelet aggregation
Once the mRNA is translated, it is formed a premature protein called Pre-pro-VWF made up of a signal peptide of 22 aa, a propeptide of 741 aa and a mature subunit of 2050 aa. The pro-VWF, formed by the propeptide and mature subunit, is composed of 4 types of domains that are repeated along the protein and carrying out the various functions that allow the platelet aggregation
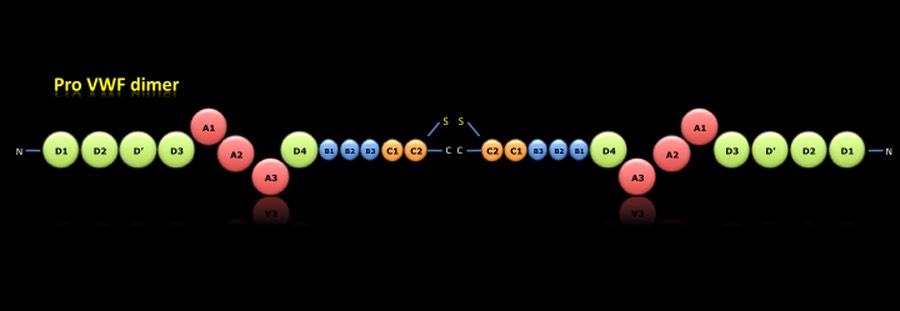
PRO-VWF DIMER:
During the intracellular synthesis, within the endoplasmic reticulum, there are different posttranslational processes. Once removed the signal peptide were formed the pro-VWF dimers by disulfide bridges in the carboxy-terminal region.
During the intracellular synthesis, within the endoplasmic reticulum, there are different posttranslational processes. Once removed the signal peptide were formed the pro-VWF dimers by disulfide bridges in the carboxy-terminal region.
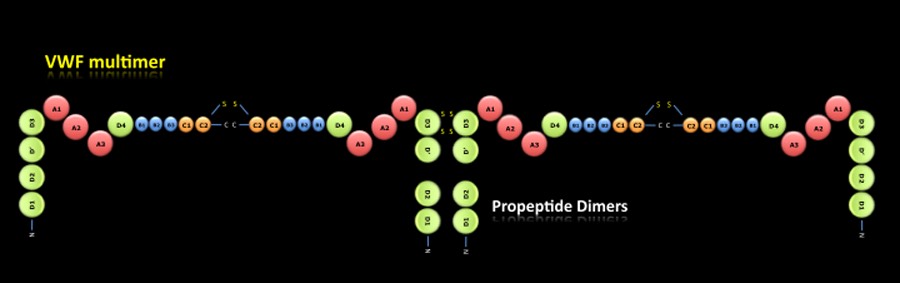
VWF MULTIMER:
The pro-VWF dimers are transported to the Golgi apparatus where the domains D1, D2, D' and D3 are associated between them forming pro-VWF multimers. Through a proteolytic process, the propeptide dimers (Domains D1 and D2) are released and mature VWF multimers are obtained which will be stored in the Weibel-Palade bodies of endothelial cells and alpha-granules of platelets.
The pro-VWF dimers are transported to the Golgi apparatus where the domains D1, D2, D' and D3 are associated between them forming pro-VWF multimers. Through a proteolytic process, the propeptide dimers (Domains D1 and D2) are released and mature VWF multimers are obtained which will be stored in the Weibel-Palade bodies of endothelial cells and alpha-granules of platelets.
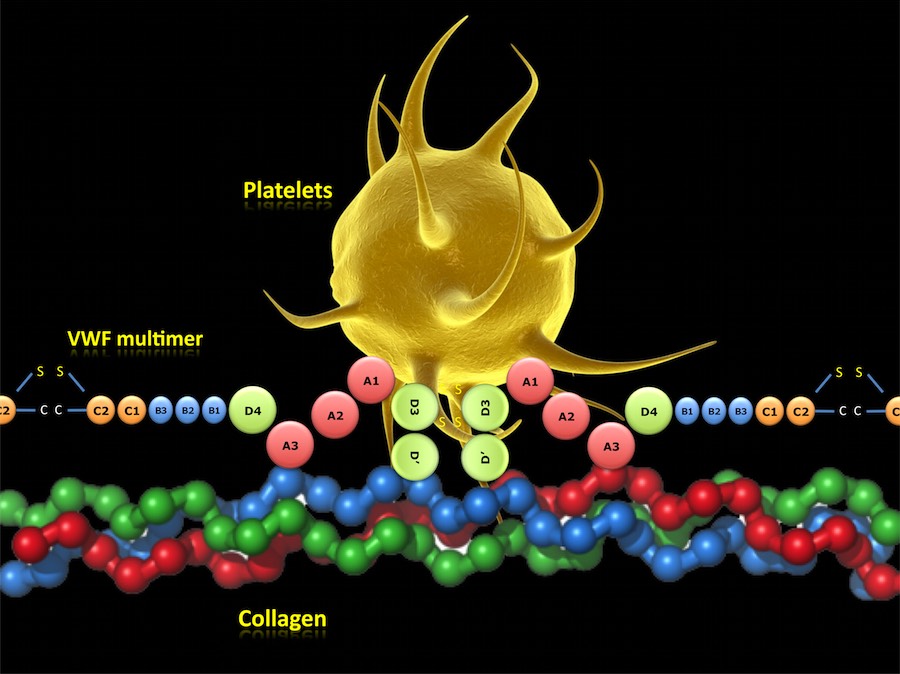
VWF MULTIMER FUNCTIONS:
The VWF has three main functions: promotes the adhesion of platelets to subendothelial structures at the site of vascular injury, protects FVIII from inactivation and rapid catabolism and promotes platelet-platelet interaction.
In case of injury, the VWF multimers become immobilized via its A3 domain on the fibrillar collagen of the vessel wall and acts as an intermediary between collagen and the platelet receptor GPIbα. After GPIbα binds to the A1 domain of its main ligand VWF, further activation of the platelet via intracellular signalling occurs, allowing other receptors to engage VWF and collagen and thereby reinforcing permanent adhesion.
The VWF has three main functions: promotes the adhesion of platelets to subendothelial structures at the site of vascular injury, protects FVIII from inactivation and rapid catabolism and promotes platelet-platelet interaction.
In case of injury, the VWF multimers become immobilized via its A3 domain on the fibrillar collagen of the vessel wall and acts as an intermediary between collagen and the platelet receptor GPIbα. After GPIbα binds to the A1 domain of its main ligand VWF, further activation of the platelet via intracellular signalling occurs, allowing other receptors to engage VWF and collagen and thereby reinforcing permanent adhesion.